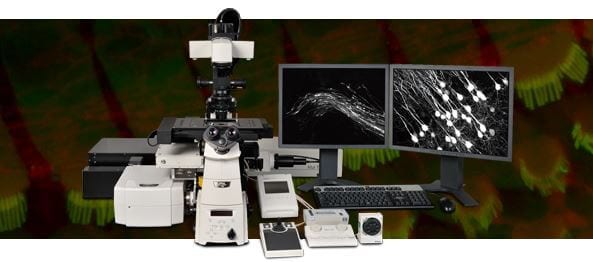
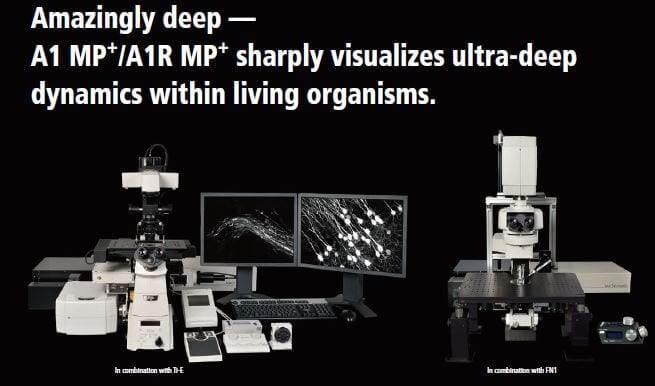
Faster, deeper, sharper multiphoton confocal imaging
New multiphoton and confocal microscope system for high speed, high resolution and high sensitivity multiphoton excitation and confocal fluorescence imaging.
Nikon’s A1R MP+ multiphoton confocal microscope is a unique multiphoton imaging system featuring a fast, high resolution galvanometer scanner and an ultra-high speed resonant scanner that is capable of frame rates from 30 fps at 512 x 512 pixels to as fast as 420 fps in band scan mode. This is especially important in multiphoton microscopy because of the overlap of emission spectra of probes and autofluorescence, which is often unavoidable when using a single laser line.
Nikon’s exclusive resonant scanner is capable of imaging a wide area at a much higher speed than a non-resonant scanner, making it possible for 420-fps imaging using point scanning technology. The NDD for multiphoton microscopy makes it possible to image fast and deep through the thickest specimens. Nikon’s optical pixel clock system allows more stable and more evenly illuminated imaging— even at high speeds.
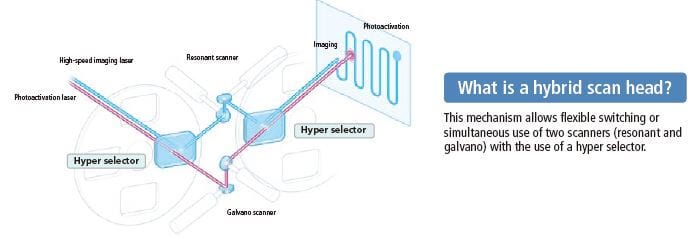
The A1R+ has a hybrid scanner head that incorporates both an ultrahigh-speed resonant scanner and a high-resolution galvano scanner. Simultaneous photoactivation and ultrafast imaging using these two scanners allow acquisition of rapid changes after photoactivation and enables observation of intermolecular interaction.
The Nikon resonant scanner is capable of high-speed 420-fps imaging. Unique to this design is a resonant scan mirror capable of imaging full fields of view at much higher speeds than traditional galvano scanners. Nikon’s optical pixel clock system, which monitors the position of the resonant mirror in real time, adjusts the pixel clock to ensure more stable, geometrically correct and more evenly illuminated imaging even at high speeds. This enables the successful visualization of in vivo rapid changes, such as reactions in living organisms, dynamics and cell interactions.
The fluorescence emissions from deep within a specimen are highly scattered in multiphoton excitation, and therefore the conventional detector using a pinhole cannot provide bright fluorescent images. The episcopic NDD in the A1R MP+ is located close to the back aperture of the objective to detect the maximum amount of scattered emission signals from deep within living specimens.
The cerebral cortex of an H-line 5-week-old mouse was studied with the open skull method. The entire shape of dendrites of pyramidal cells in layer V expressing EYFP were visualized from the bottom layer into a superficial layer. In addition, the fluorescence signal of white matter in deeper areas was also studied.

| Left | 3D reconstruction image | Photographed with the cooperation of:Dr. Tomomi Nemoto, Research Institute for Electronic Science, Hokkaido UniversityDr. Shigenori Nonaka, National Institute for Basic BiologyDr. Takeshi Imamura, Graduate School of Medicine, Ehime University |
|
| Right | Z-stack images | ||
| Top: dendrites located in superficial layers in the layer V pyramidal cells 25 µm from the surface |
|||
| Middle: basal dendrites in the layer V pyramidal cells 625 µm from the surface |
|||
| Bottom: fluorescence from white matter Excitation wavelength: 950 nm Objective: CFI75 Apo 25xW MP (NA 1.10 WD 2.0) |
Mouse cerebral cortex multi-color imaging
|
|
|
|
Simultaneous acquisition of three channels in anesthetized YFP-H mouse using IR excitation of 950 nm and imaging Second Harmonic Generation (SHG) and two fluorescence emissions.
Cyan: SHG signal of dura mater
Yellow: EYFP pyramidal neurons in layer V of the cortex
Red: SRB-labeled blood vessels
Photographed with the cooperation of:
Drs. Ryosuke Kawakami, Terumasa Hibi and Tomomi Nemoto, Research Institute for Electronic Science, Hokkaido University
3D volume rendering images
Three-dimensional volume renderings of a kidney labeled with Hoxb7/myrVenus marker (Chi et al, 2009 Genesis), using depth-code pseudocolor volume rendering to reference Z depths (pseudocolored by depth – 1 μm step for 550 μm).

Objective: CFI Apochromat 25xW MP, Scan zoom: 1x, Z step size: 1 μm, IR excitation wavelength: 930 nm
Image resolution: 1024×1024 pixels, Image volume: 460 μm (length) x 460 μm (width) x 600 μm (height)
Photographed with the cooperation of Dr. Frank Costantini and Dr. Liza Pon, Columbia University Medical Center, New York
In addition to the GaAsP NDD compatible with a wavelength of 1080nm, there is a new model for upright microscopes that is compatible with a wavelength of 1300 nm. This new NDD enables deep imaging up to 1.4 mm in combination with a newly developed scanning head A1R MP+ that is compatible with a wavelength of 1300 nm.
Deep brain imaging in in vivo mouse with the GaAsP NDD compatible with the 1300 nm wavelength
In vivo imaging of an anesthetized YFP-H mouse (4-week-old) via open skull method. Visualization of the entire layer V pyramidal neurons and the deeper hippocampal neurons. Deep imaging achieved for 3-dimensional imaging of hippocampal dendrites up to 1.4 mm into the brain.
Captured with episcopic GaAsP NDD for 1300 nm and CFI75 Apochromat 25xW MP1300 objective lens (NA 1.10, WD 2.0 mm)
Excitation wavelength: 1040 nm
Mouse brain in vivo dual color imaging with the GaAsP NDD compatible with the 1300 nm wavelength
The cerebral cortex of an anesthetized YFP-H mouse (4-week-old) was studied with the open skull method.
Alexa594 was injected into the tail vein to visualize the blood vessel.
With the dual IR beam option, users can now simultaneously excite two different fluorophores such as GFP and mCherry. This capability enables ultra-fast two-color multiphoton imaging, ideal for dynamic specimens. The dual beams can also be used for stimulation at a specific wavelength and imaging with a different wavelength, reducing time delays normally present when using a single beam which requires mode locking to change wavelengths.

Live brain slice expressing YFP and Rhod-2, simultaneously excited with 900nm and 1040nm wavelengths (CFI75 LWD Apo 25x 1.1 NA W 1300nm objective). A live slice culture from a YFP-VGAT rat stained with rhod-2 AM was imaged to measure mitochondrial calcium transients in hippocampal interneurons.
High-NA objectives have been developed that highly correct chromatic aberrations over a wide wavelength range, from ultraviolet to infrared. Transmission is increased through the use of Nikon’s exclusive Nano Crystal Coat technology.
In particular, the CFI75 Apochromat 25xW MP/MP1300 objective lenses provide an industry leading highest numerical aperture of 1.10 while still maintaining a 2.0 mm working distance. They also have a collar that corrects spherical aberrations depending on the depth of the specimen and a 33° manipulator pipette access angle, making it ideal for deep multiphoton imaging and physiology research applications.
Nano Crystal Coat is a Nikon exclusive lens coating technology using an ultralow refractive index nanoparticle thin film originally developed for the semiconductor fabrications industry. The Nano Crystal Coat particle structure dramatically reduces stray reflections and boosts transmission over a wide wavelength range, producing images with higher signal-to-noise (S/N) ratios.
| CFI75 Apochromat 25xW MP1300 |
NA 1.10 WD 2.0 Nano Crystal Coat |
|---|---|
| CFI75 Apochromat 25xW MP |
NA 1.10 WD 2.0 Nano Crystal Coat |
| CFI Apochromat LWD 20xWI λS |
NA 0.95 WD 0.95 Nano Crystal Coat |
| CFI Apochromat LWD 40xWI λS |
NA 1.15 WD 0.6 Nano Crystal Coat |
| CFI Apochromat 40xWI λS |
NA 1.25 WD 0.18 Nano Crystal Coat |
| CFI Plan Apochromat IR 60xWI |
NA 1.27 WD 0.17 Nano Crystal Coat |
When the multiphoton laser wavelength or group velocity dispersion pre-compensation is changed, the multiphoton laser beam positional pointing at the objective back aperture may also change, resulting in uneven intensity across the image, or a slight misalignment between the IR and visible laser light paths.
Verifying the IR laser beam pointing and setting the alignment has traditionally been difficult. The A1R MP+ auto laser alignment function, housed in the Incident Optical Unit for the multiphoton excitation light path, automatically maximizes IR laser alignments with a single click in NIS-Elements C.
A1R MP+ has a hybrid scanning head that incorporates both a high-resolution galvano scanner and an ultrahigh-speed resonant scanner. The galvano scanner enables imaging up to 4096 x 4096 pixels and high-speed acquisition of 10 fps (512 x 512 pixels). A new A1R MP+ system is now available which is compatible with a wavelength of 1300 nm.
| Scan head input/output port | 3 laser input ports 3 signal output ports for standard, spectral and optional detector*1 |
|
|---|---|---|
| Laser for multiphoton microscopy | Compatible laser | Mai Tai HP/eHP DeepSee, InSight DS+*2, InSight DS+ Dual Option*3 (Spectra-Physics) Chameleon Vision Ⅱ, Chameleon Discovery*3 (Coherent) |
| Modulation | Method: AOM (Acousto-Optic Modulator) device Control: power control, return mask, ROI exposure control |
|
| Incident optics | 700 – 1080 nm*4 *5, 700 – 1300 nm*2 *3, auto alignment | |
| Laser for confocal microscopy (option) | LU-N3 3 laser unit | 405 nm, 488 nm, 561 nm laser are installed; built-in AOTF
*Cannot used with spectral detector. |
| LU-N4/LU-N4S 4laser unit | 405 nm, 488 nm, 561 nm, 640 nm laser are installed; built-in AOTF
*Use LU-N4S when using the spectral detector. |
|
| LU-NV Laser unit | Compatible laser: 405 nm, 445 nm, 458 nm, 488 nm, 514 nm, 532 nm, 561 nm, 594 nm, 640 nm, 647 nm; built-in AOTF | |
| NDD for multiphoton microscopy | Type | Compatible with 1080 nm Episcopic GaAsP NDD (for Ni-E/FN1/Ti-E), Diascopic GaAsP NDD (for Ni-E, FN1), Diascopic NDD (for Ni-E only): Detectable wavelength range 380 – 650 nm*6 |
| Episcopic GaAsP NDD compatible with 1300 nm (for Ni-E/FN1) : Detectable wavelength range 380 – 750 nm | ||
| Detector | 4 PMTs (3 GaAsP PMTs and 1 normal PMT for GaAsP NDD) |
|
| Filter cube | 450/50, 450/70, 492, 525/50, 550/88, 575/25, 610/75, 629/53, 641/75, 732/68 | |
| Standard fluorescence detector (option) | Detectable wavelength range | 400 – 750 nm (400 – 650 nm when using IR laser) |
| Detector | A1-DU4 4 Detector Unit: 4 normal PMTs A1-DUG GaAsP Multi Detector Unit: 2 GaAsP PMTs + 2 normal PMTs |
|
| Filter cube | 6 filter cubes commonly used for a microscope mountable on each of three filter wheels Recommended wavelengths for multiphoton/confocal observation: 450/50, 482/35, 515/30, 525/50, 540/30, 550/49, 585/65, 594LP, 595/50, 700/75 |
|
| Diascopic detector (option) | Detectable wavelength range | 440 – 645 nm |
| Detector | PMT | |
| FOV | Square inscribed in a Φ18 mm circle | |
| Image bit depth | 4096 gray intensity levels (12 bit) | |
| Scan head | Type | A1-SHRM (compatible with 1080 nm)/A1-SHRMV (compatible with 1080 nm and visible light photoactivation/IR imaging)/A1-SHRM3 (compatible with 1300 nm)/ A1-SHRMD (compatible with 1300 nm and Dual IR) |
| Standard image acquisition | Scanner: galvano scanner x2 Pixel size: max. 4096 x 4096 pixels Scanning speed: Standard mode: 2 fps (512 x 512 pixels, bi-direction), 24 fps (512 x 32 pixels, bi-direction) Fast mode: 10 fps (512 x 512 pixels, bi-direction), 130 fps (512 x 32 pixels, bi-direction) *7 Zoom: 1-1000x continuously variable Scanning mode: X-Y, X-T, X-Z, XY rotation, Free line |
|
| High-speed image acquisition | Scanner: resonant scanner (X-axis, resonance frequency 7.8 kHz), galvano scanner (Y-axis) Pixel size: max. 512 x 512 pixels Scanning speed: 30 fps (512 x 512 pixels) to 420 fps (512 x 32 pixels), 15,600 lines/sec (line speed) Zoom: 7 steps (1x, 1.5x, 2x, 3x, 4x, 6x, 8x) Scanning mode: X-Y, X-T, X-Z Acquisition method: Standard image acquisition, High-speed image acquisition, Simultaneous photoactivation and image acquisition |
|
| IR laser wavelength range | 700 – 1080 nm*4 *5, 700 – 1300 nm*2 *3 | |
| Dichroic mirror | Low-angle incidence method Position: 8 Standard filter: 405/488, 405/488/561, 405/488/561/638, 400 – 457/514/IR, 405/488/543/638, IR total reflection*2 *3, BS20/80, IR, 405/488/561/IR |
|
| Pinhole | 12 – 256 μm variable (1st image plane) | |
| Spectral detector*8(option) | Detectable wavelength range | 400 – 750 nm (400 – 650 nm when using IR laser) |
| Number of channels | 32 channels | |
| Spectral image acquisition speed | 4 fps (256 x 256 pixels), 1000 lps | |
| Wavelength resolution | 80 nm (2.5 nm), 192 nm (6 nm), 320 nm (10 nm) Wavelength range variable in 0.25 nm steps |
|
| Unmixing | High-speed unmixing, Precision unmixing | |
| Compatible microscopes | ECLIPSE Ti-E inverted microscope, ECLIPSE FN1 fixed stage microscope, ECLIPSE Ni-E upright microscope (focusing nosepiece type) | |
| Z step | Ti-E: 0.025 μm, FN1 stepping motor: 0.05 μm Ni-E: 0.025 μm |
|
| Option | Motorized XY stage (for Ti-E/Ni-E), High-speed Z stage (for Ti-E), High-speed piezo objective-positioning system (for FN1/Ni-E) | |
| Software | Display/image generation | 2D analysis, 3D volume rendering/orthogonal, 4D analysis, spectral unmixing |
| Image format | JP2, JPG, TIFF, BMP, GIF, PNG, ND2, JFF, JTF, AVI, ICS/IDS | |
| Application | FRAP, FLIP, FRET (option), photoactivation, three-dimensional time-lapse imaging, multipoint time-lapse imaging, colocalization | |
| Control computer | OS | Microsoft Windows®7 Professional 64 bit SP1 |
| CPU | Intel Xeon E5-2667 (2.90G.Hz/15MB/1600MHz) | |
| RAM | 16 GB (4GB x4) | |
| Chipset | Intel® C602 chipset | |
| HDD | 300 GB SAS 3GB/s (15,000 rpm) x2, RAID-0 configuration | |
| Graphic controller | NVIDIA Quadro 600 (PCI Express/dual monitor), or NVIDIA Quadro 4000 (PCI Express/dual monitor) |
|
| Optical drive | Super multi-drive, max. 16x or faster | |
| Extension slot | PCI slot x1 PCI Express 3.0 (x16) x3 (for graphics) PCI Express 3.0 (x16 mechanical, x8 electrical) x1 PCI Express 3.0 (x8 mechanical, x4 electrical) x1 PCI Express 2.0 (x8 mechanical, x4 electrical) x1 |
|
| Network interface | Serial x1, RJ45 | |
| LAN port | 10/100Base-T 1 port (for controller) 10/100/1000Base 1 port (for I/F board for external LAN) |
|
| Monitor | 1600 x 1200 or higher resolution, dual monitor configuration recommended | |
| Vibration isolated table | 1800 (W) x 1500 (D) mm required (for FN1/Ni-E), or 1500 (W) x 1500 (D) mm required (for Ti-E) | |
*1 FCS/FCCS/FLIM is possible in combination with third-party systems.
*2 When using a scan head compatible with 1300 nm.
*3 When using a scan head compatible with 1300 nm and Dual IR.
*4 When using a scan head compatible with 1080 nm.
*5 When using a scan head compatible with 1080 nm and visible light photoactivation/IR imaging.
*6 400 – 650 nm when using diascopic NDD.
*7 Fast mode is compatible with zoom 8-1000x and scanning modes X-Y and X-T. It is not compatible with Rotation, Free line, CROP, ROI, Spectral imaging, Stimulation and FLIM.
*8 Compatible with galvano scanner only